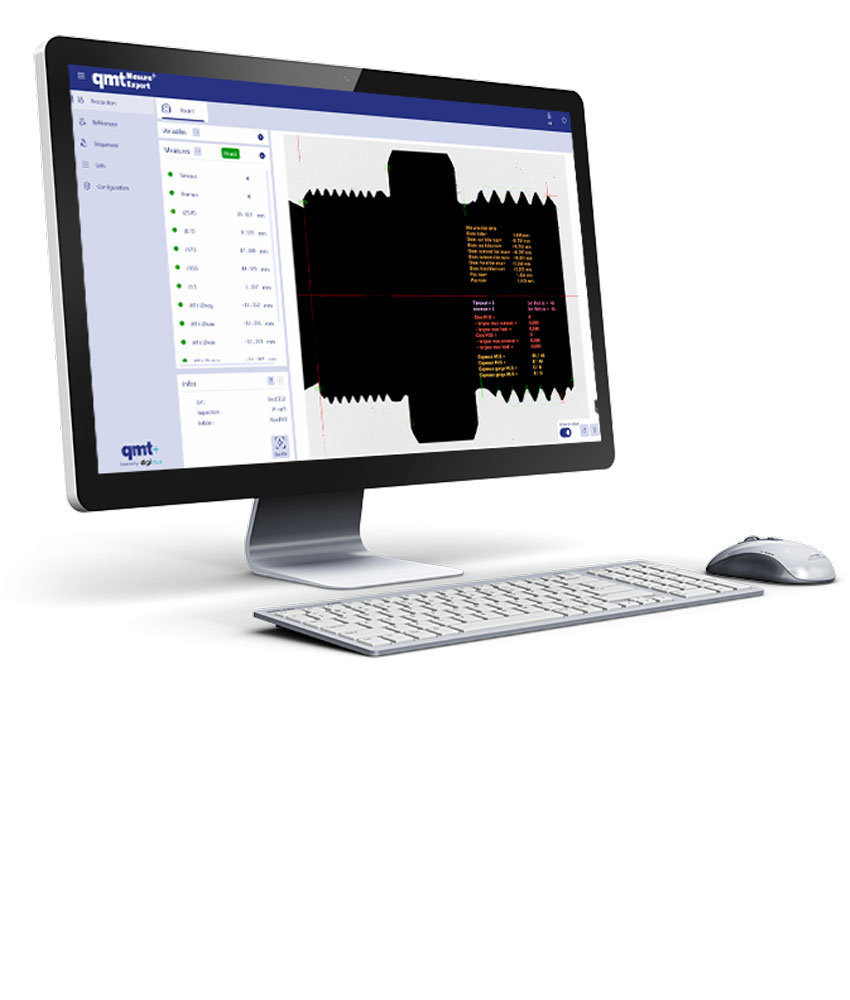
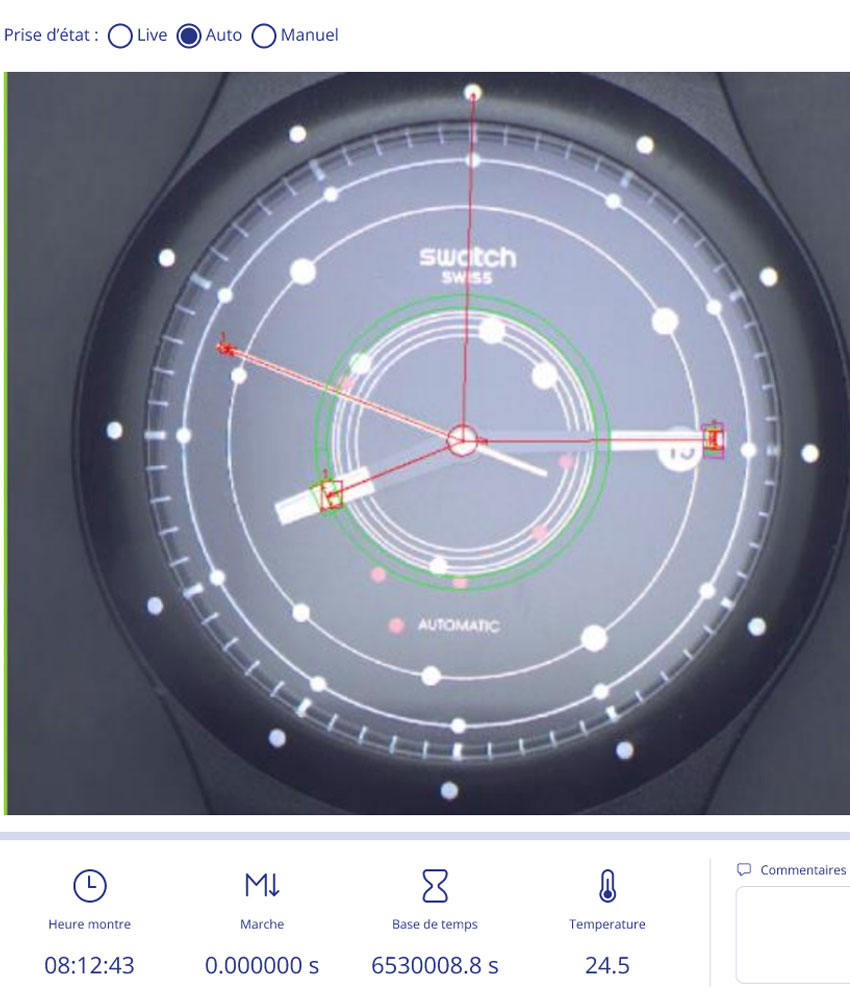
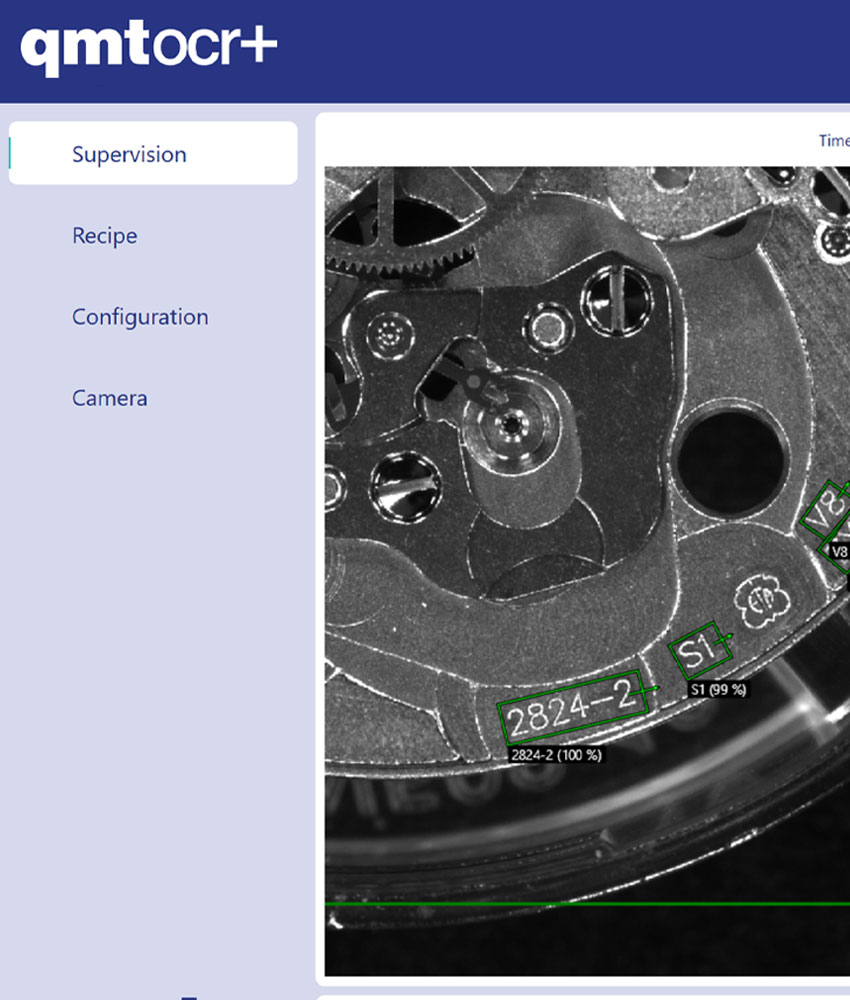
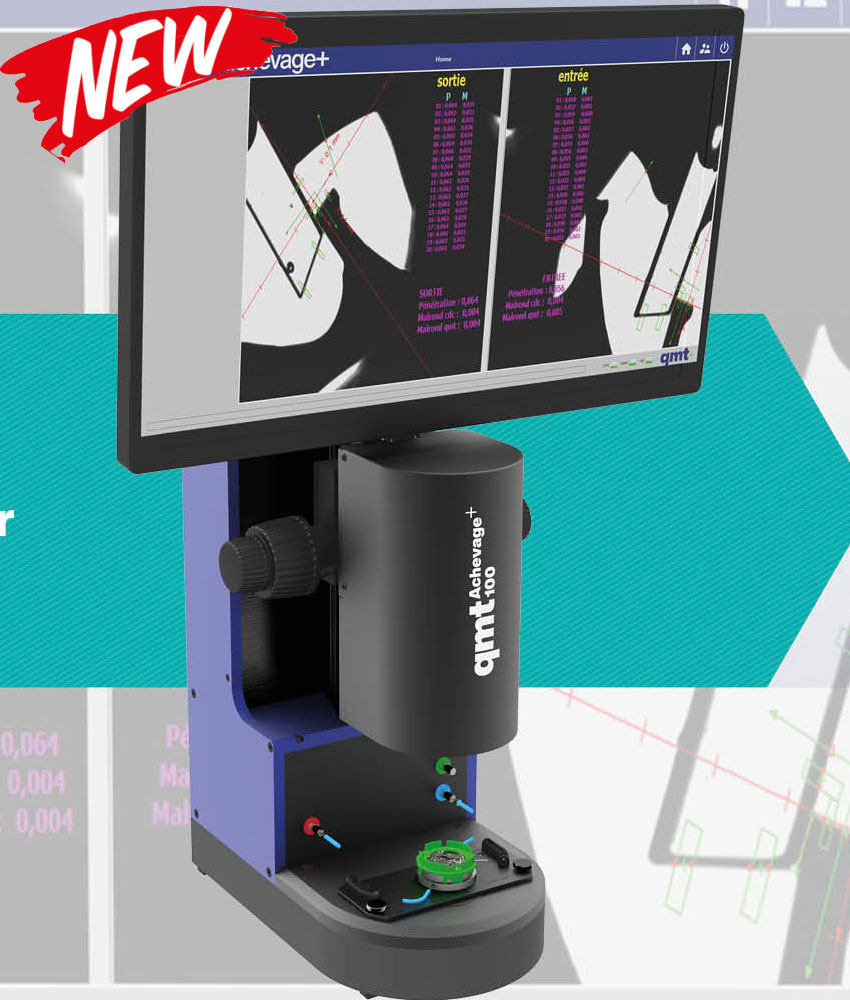
Actualités
QMT receives medical certification to ISO 13485
This result underlines the company's commitment in the medical world for over 10 years

Since 1989, a presence in the medical market, QUALIMATEST leader in the production of quality control equipment integrated vision systems, its development has always been focused on technology. And if his growth was almost 25 consecutive years, she wants to accelerate to a variety of companies and business units to provide technology solutions. This market represents more than 21% of the activity of the Qualimatest.
With the aim of the quality requirements of its customers and regulations to better meet further Qualimatest continuous improvement of processes and adapted its quality management in the medical world needs.
According to the ISO 9001 certification in 2011 obtained QUALIMATEST more to obtain the certification ISO 13485 Ivan Meissner, CEO of QUALIMATEST open their doors this leading sector, said: "This important step in the recognition of our quality organization demonstrates our ability to medical projects in line with customer requirements and applicable regulations to manage. "
Particular attention is given to the risk analysis in the development, traceability and materiovigilance of vision systems to the medical sector. Along the value chain, the Qualimatest products are designed, developed and manufactured to be with the goal of safer and more effective.
About ISO 13485
The ISO 13485 internationally recognized is the repository for the implementation of quality management approaches. It applies to companies that design, manufacture, repair and sale of medical products, but also to systems controls this technology products.