Réalisations
Bandage checking machine
ISO13485 certified since 2014, qmt provides testing and quality control solutions for the medical industry. The growing share of Medtech customers represents 17% of sales in 2019.

A custom-developed control device
qmt supported its client in setting up an automated quality control for a woven component at the end of production. High added value and innovative products must be 100% checked at the end of production to ensure that they are free from defects.
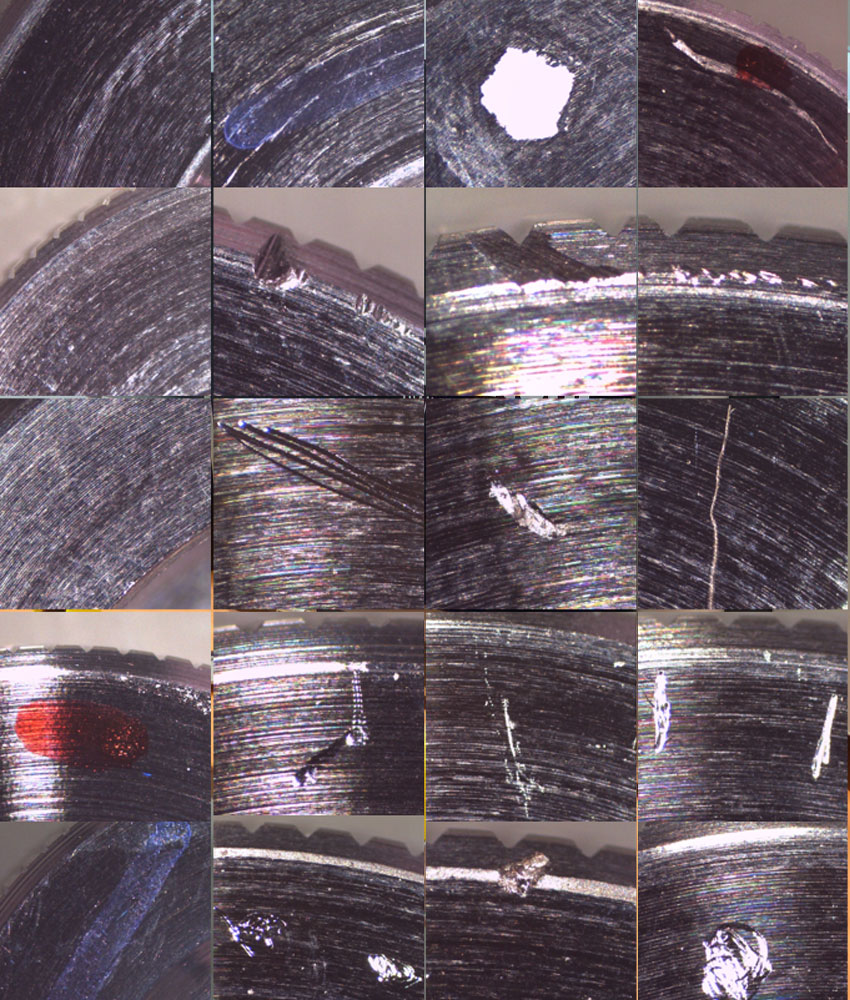
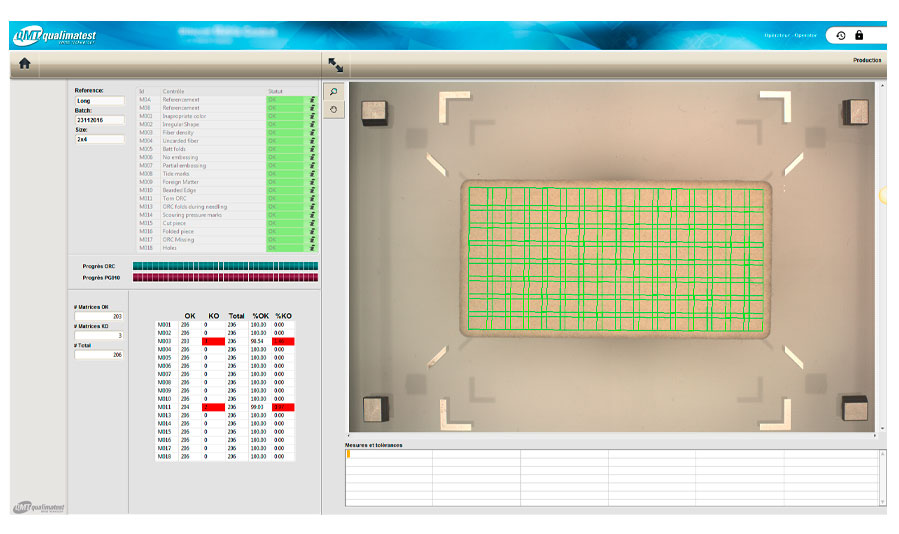
18 different types of defects are sought: poor weaving density, pollution, colors, absence of holes, ...
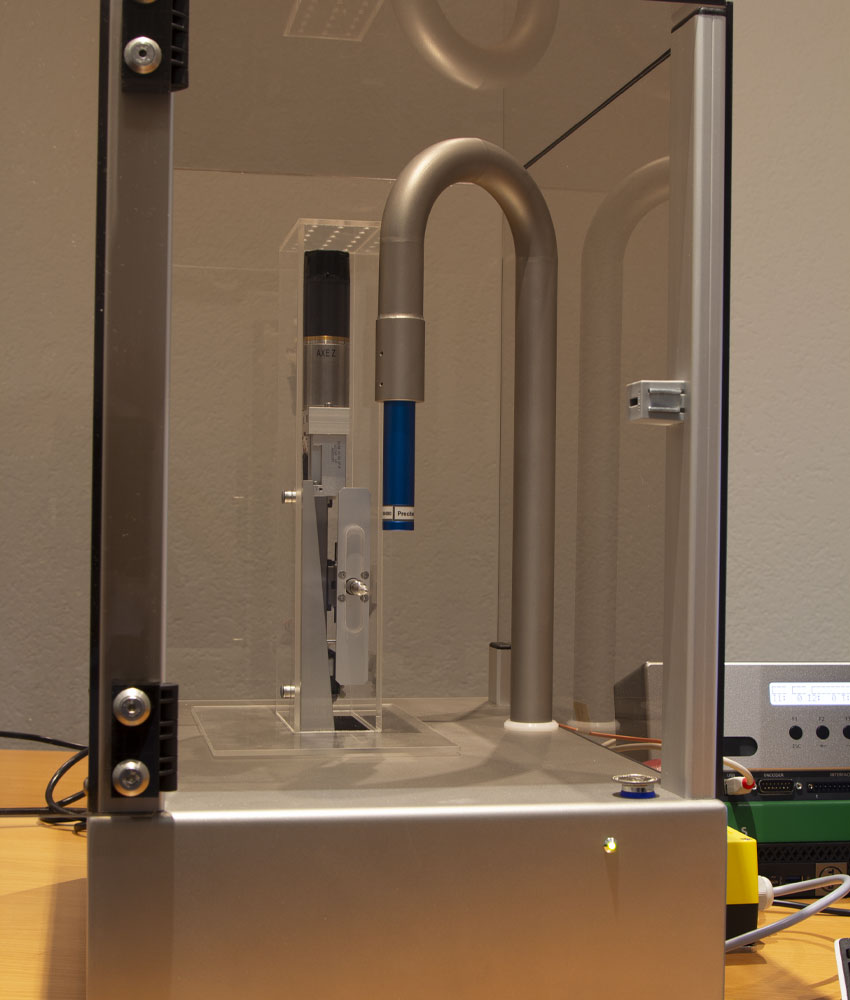
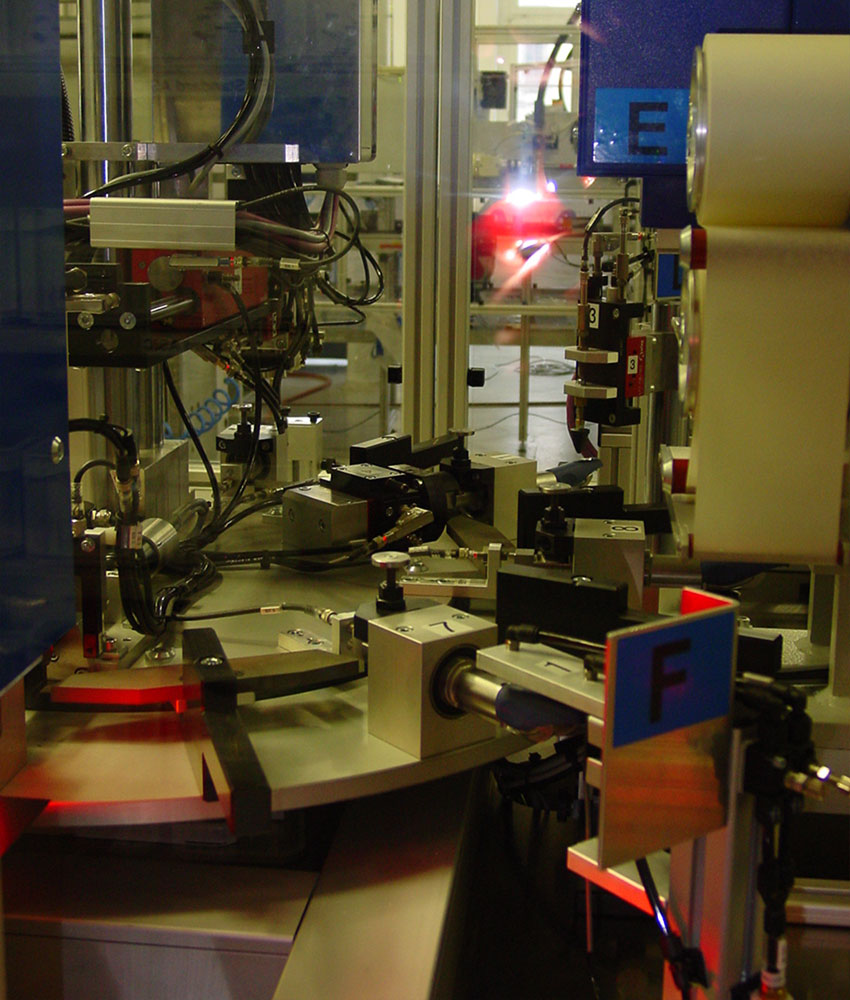
A visual inspection operation would not have been able to guarantee the robustness of the inspection. qmt has therefore developed a semi-automatic control device: product handling is manual but all controls are automated.
To perform the optical checks, the device incorporates a very high resolution camera and 6 different lights.
The control device has been developed in accordance with the ISO1385 medical standard including all the validation phases which have been managed by qmt .
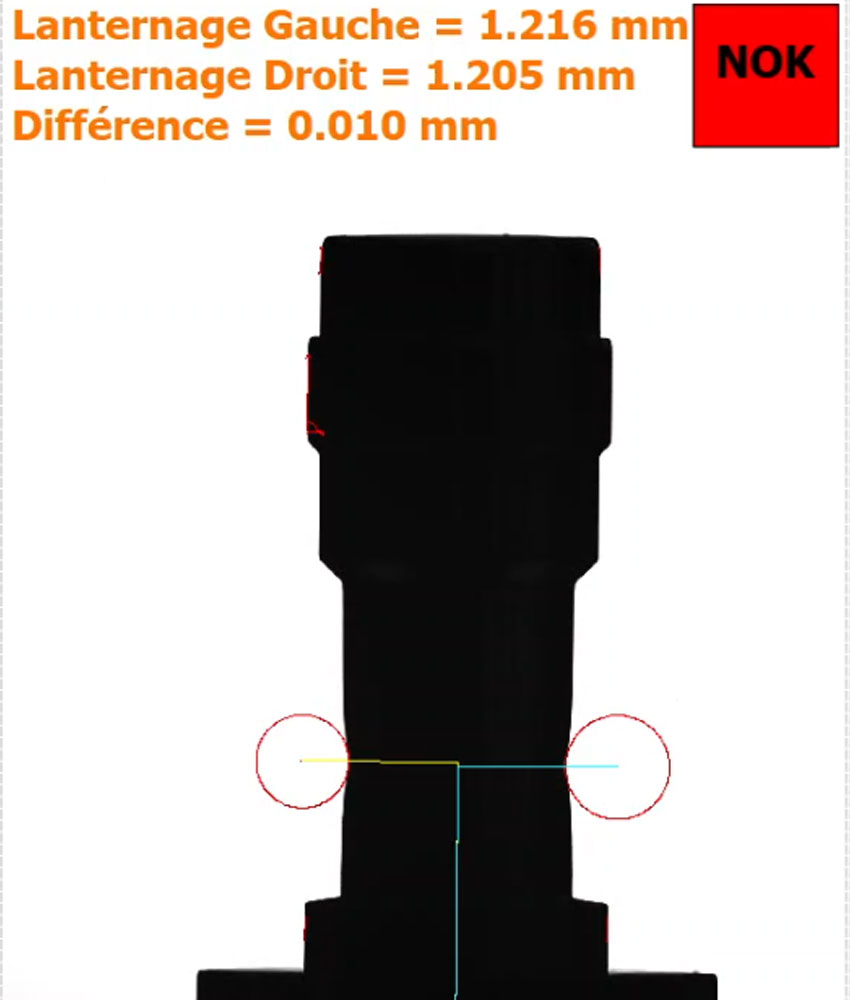
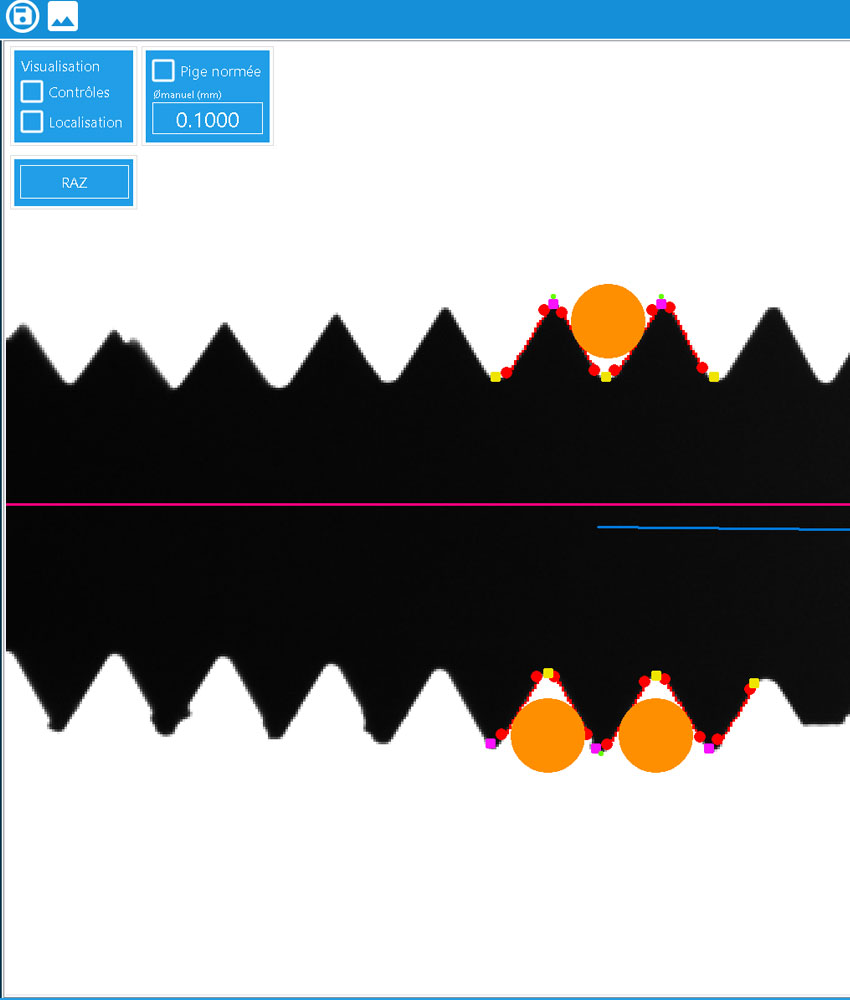
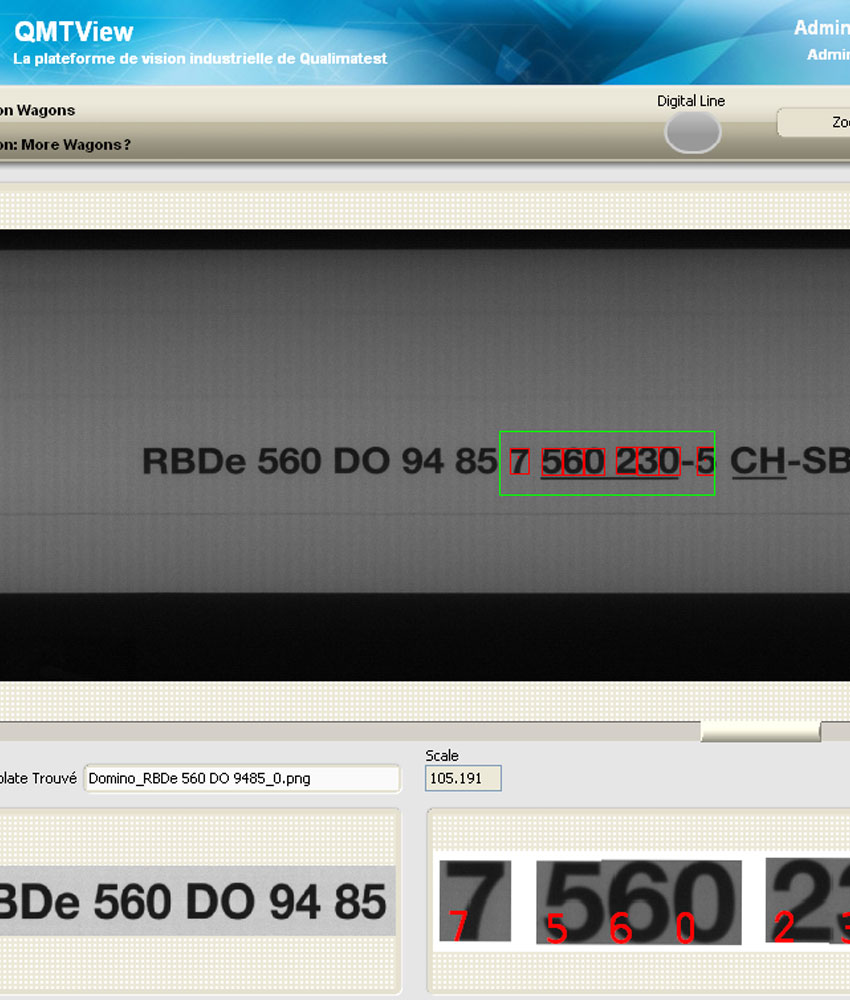
- 18 checks carried out on the product in seconds
- Very high control resolution: 4,900 x 3,200 pixels
- Environment and control conditions strongly controlled with 6 different lights activated according to a defined cycle
- ISO 13485 certified equipment
A new generation dressing which is a "fabric" measuring 50 x 100mm. The checks to be carried out:
- Color and its uniformity
- Regularity of form
- Fiber density
- Fiber structure: shape, hole and uniformity
- No presence of particles and pollution
A tailor-made device was developed, manufactured, assembled and perfected by the qmt team. It allows the motorized loading of the product to be controlled and the control of the control conditions. It is particularly important to master the optical, camera and lighting conditions.
In order to achieve the expected performance, a cutting-edge optical solution containing 6 lights has been developed.
The optical solution defined by the qmt multi-inspection team has been completely integrated by the mechatronics team in order to provide a turnkey tester to the customer.
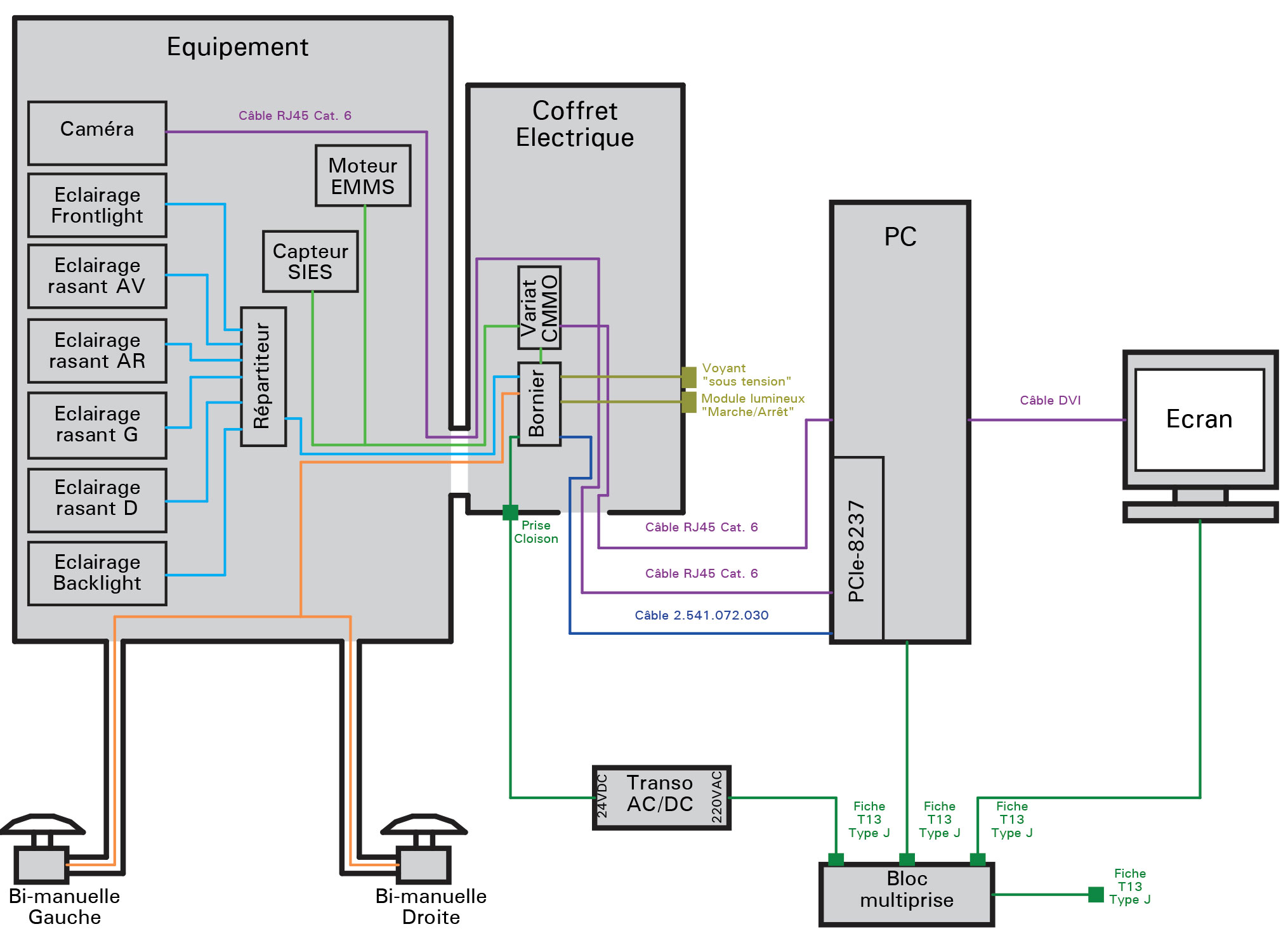
The aesthetic checks to be carried out have acceptance limits that cannot be easily defined quantitatively. In order to define the feasibility of the controls, their limits and to quantify the tolerances, a detailed study formalized by a report has to be carried out. This study made it possible to finalize the URS to define the technical solution and the costs. On this basis qmt made a commitment on the specifications, the price and the completion time.
The qmt software team has developed specific software for this tester. This software integrates an easy-to-use user interface while allowing the realization of all the controls. The software has been validated according to medical standards and ISO13485 (Validations IQ, OQ and PQ).
The multi-disciplinary capabilities of qmt allowed the realization of this turnkey control device. The ISO13485 certified qmt organization made it possible to meet the customer's regulatory requirements.